
SÜDPACK Newsletter
Receive regular updates about SÜDPACK, our products and sustainability topics.

Receive regular updates about SÜDPACK, our products and sustainability topics.
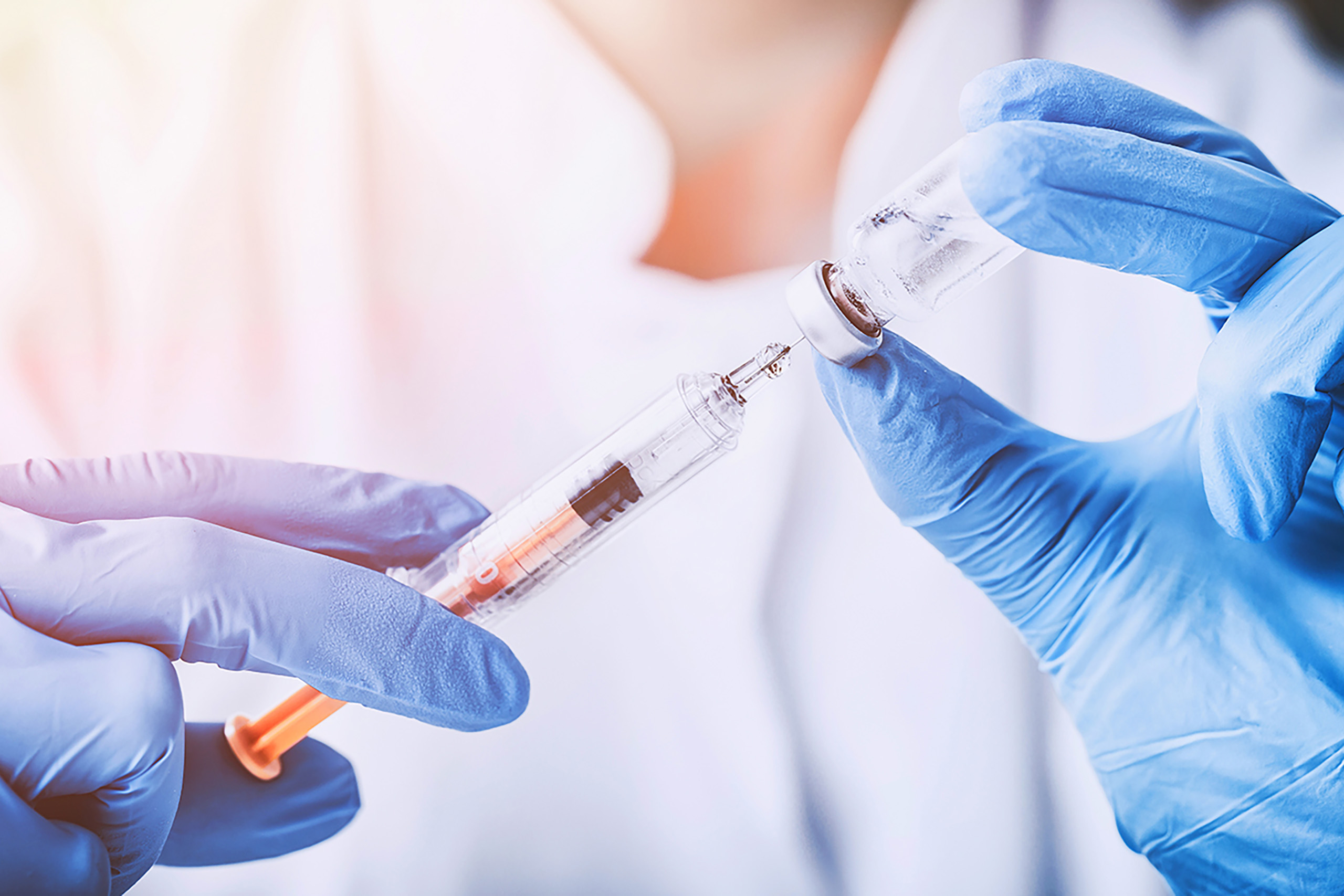
Drug products are subject to stringent regulations – from development, manufacture and storage, right up to their application.
This is because active ingredients, the shelf life of which is altered through environmental influences or contamination, may cause substantial health damage. Therefore, protection against such alterations and product safety are the top priority for pharmaceutical packaging.
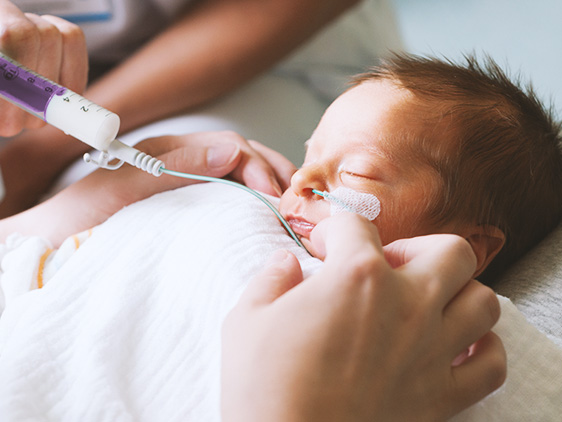
Only properly-produced tablets, coated tablets, powder or liquids are suitable for enteral application.
Our cGMP-compliant packaging films offer maximum product protection and can be processed on all common packaging machines.

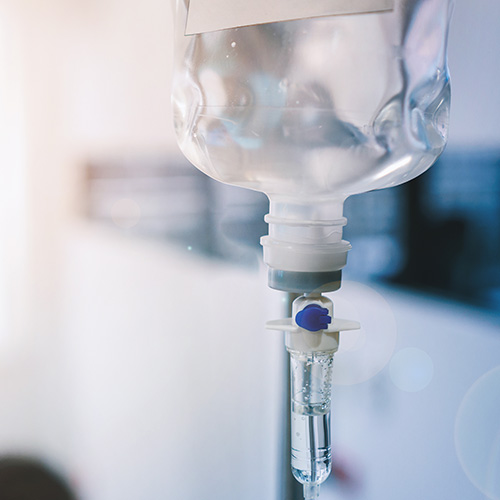
For liquid drug products intended for infusions or injections, SÜDPACK is able to offer special thermoformed films for over pouches such as tray systems, product carriers and outer packaging.
Drug products for transdermal applications or the cGMP-compliant packaging required for wound care with outstanding barrier properties.
For this purpose, SÜDPACK offers a wide spectrum of high-performance laminated films.

In the field of biopharmaceuticals, the demand for single-use systems which can be utilized as fermenters or bioreactors is increasing.
The proteins thus obtained are in turn used to obtain active ingredients such as therapeutic agents and vaccines.
Together with partners, we at SÜDPACK are developing special films for the purpose.


Receive regular updates about SÜDPACK, our products and sustainability topics.